If you haven’t already read my account of days one and two, you might like to scroll down and start at the beginning of my trip. If you have, or like to start things in the middle, read on!
Day three of my visit saw me heading for St James University Hospital in Leeds, rather than the University. After I walked past a very picturesque Victorian chapel and restored Tom met me in the Wellcome Trust Brenner Building. This University building on the hospital campus houses many of the Biology researchers and their labs.

After a lightening tour of the labs and offices, Sarah gave me an induction about the basics of lab safely and etiquette for my role as an observer. I learned where and when to wear a lab coat, what not to touch (basically everything in the lab, including not leaning or propping my notebook on any lab surfaces), when to wear gloves and when to wash hands. With all the current advice, I have already got a lot more skilled at handwashing, so that stood me in good stead.

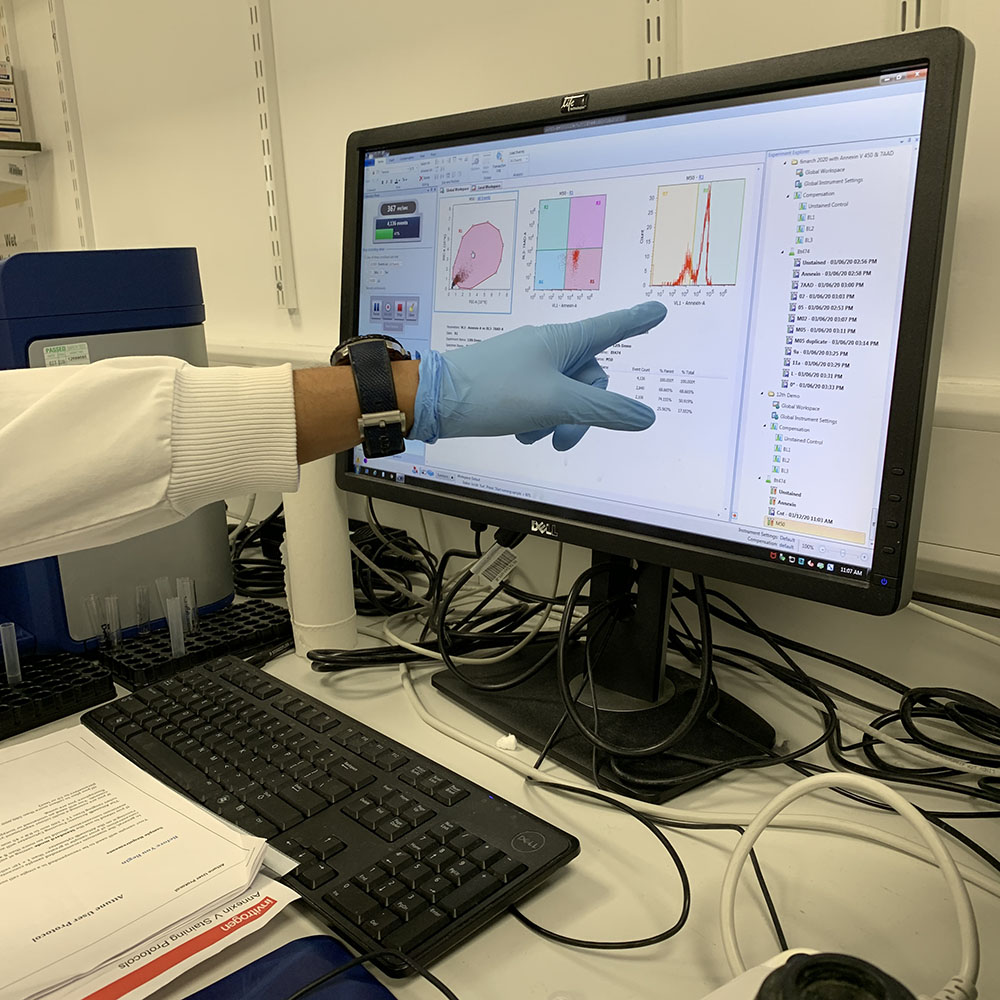
After that, I was handed into Arindam’s care. He had been prepping some experiments that we could run through the flow cytometer, part of the three day protocol that he had started before I even arrived in Leeds. The flow cytometer is an instrument which can differentiate very quickly between different cell states – which are tagged with different fluorescent labels – by passing them one by one through a narrow channel, shining a laser at them and assessing their luminosity, before chucking them back into the main sample. Amazing. Oddly, it made me think of counting sheep by making them jump over a gate.

The read out from the cytometer is shown in a graphic display on the cytometer’s monitor. Here we are looking at the percentage of cells that have died as a result of being treated with the MP1 peptide. The four box model on the screen shows living cells, early apoptosis, late apoptosis and necrosis after treatment with a particular peptide concentration.
The team are regularly working with a number of different cells line. There are four in particular that the team are using as their ‘core’ cell lines. Three of these are different breast cancer cell lines, and one is a ‘normal’ cell line (although modified so that it will continue to grow in a lab). I am really interested in how the cell lines were chosen and some of the complexities and ethics of working with cell lines, so expect to hear more about this in a separate post. My initial reaction, though, to the normal-but-immortal cell line is this: if what distinguishes a normal cell from a cancer cell is that is a cancer cell will grow and reproduce without being properly programmed to die, then the existence of a normal-but-immortal cell line is something of a conundrum – not a total contradiction but not straightforward either.

Later in the day Arindam showed me some spheroids to be treated with the peptide which we could then view through the confocal microscope to see how the peptide affected cells in a 3D configuration. In the previous experiment, the cells had been standalone, but if you are to treat cancer effectively, you are much more likely to have to treat clusters of cells. Using the normal-but-immortal cell line, Arindam had prepared the spheroids of clusters of cells – apparently the cancer cell lines don’t make good spheroids, just random bunches of cells that aren’t useful for testing.


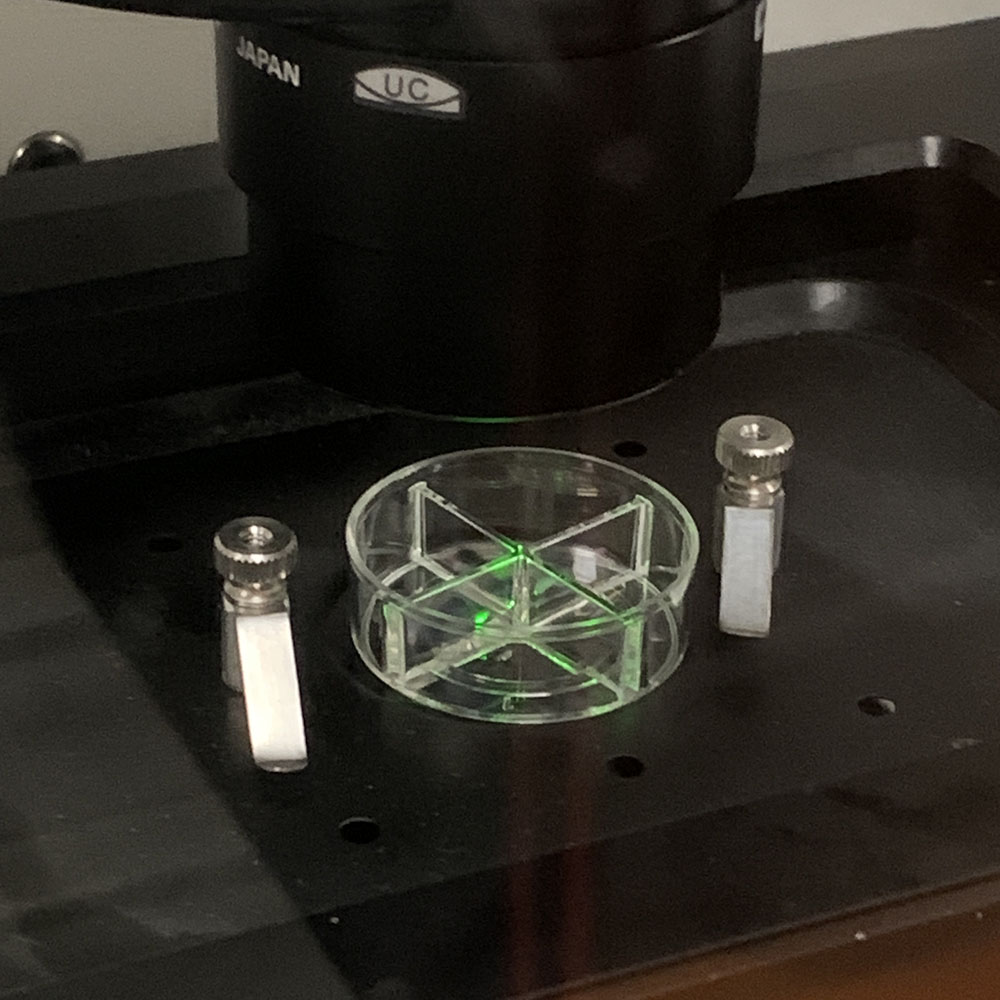
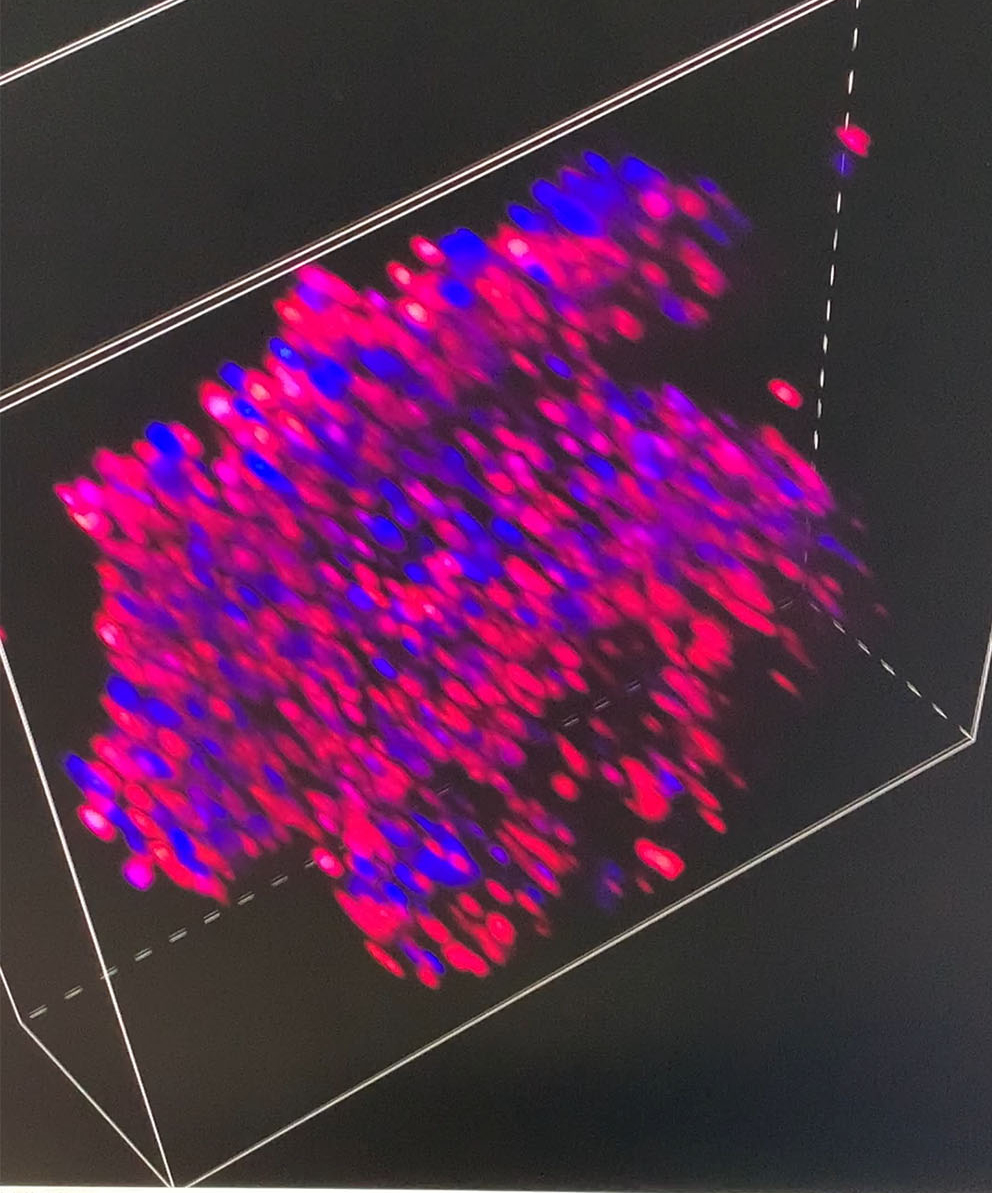
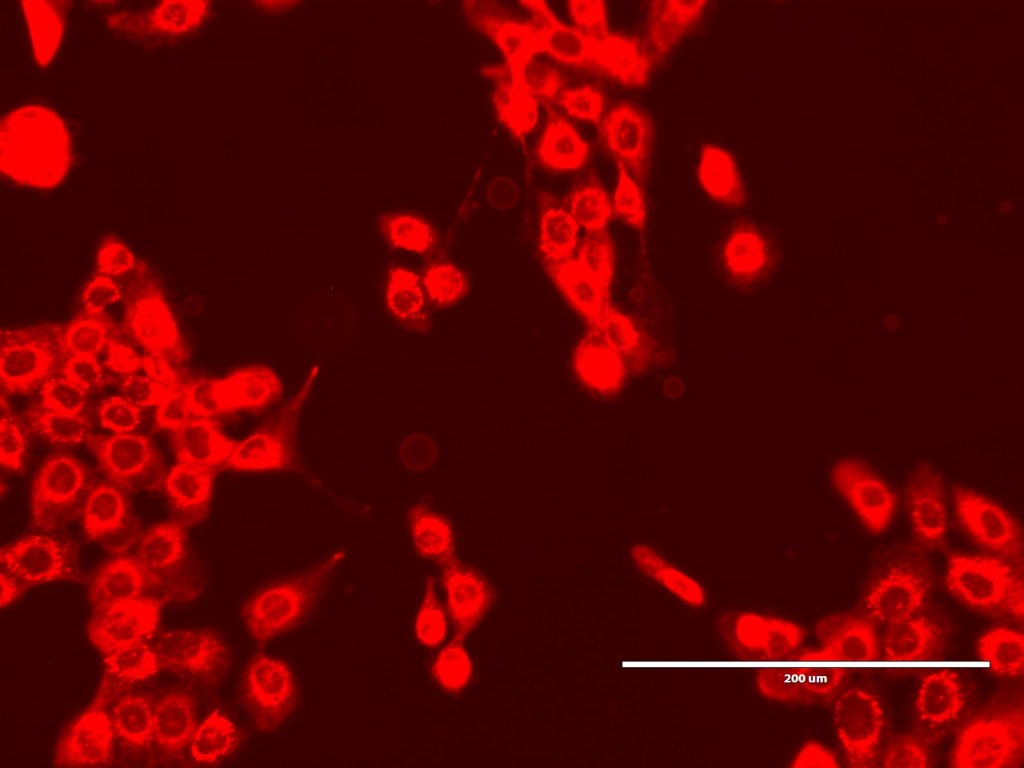
After Arindam and I had looked at various spheroids treated with different amounts of peptide, I went to spend some time with Dagmara. Using a simple optical microscope, we looked at samples of GPMVs that she had been creating from all of the different cell lines.

Dagmara is studying for her PhD as part of the project. One of her priorities so far has been to develop ways to create GPMVs that can be used as part of the peptide testing process. GPMVs are, to me, like a halfway house between testing on GUVs (or LUVs) and testing with actual cell lines. A GPMV is a vesicle created from an actual cell, so the membrane has the same (or similar) composition in terms of naturally occurring lipids etc that the cell has. It is therefore a much more complex membrane mixture than a GUV. But it doesn’t contain all the active gubbins of a real live cell, so there are fewer variables with testing GPMVs than there are with real cells.
However, it turns out that creating GPMVs – which are Giant Plasma Membrane Vesicles – are not easy things to create. Dagmara has been using two approaches – oscillation and chemical. Both techniques are tricky and oscillation only seems to work with one cell line. And even where she has been making GPMVs successfully, there are problems with them being ‘leaky’. This is a proper problem as the action of the peptide – as i understand it – is to create pores in the membrane that creates leakiness and causes the cell to expire. If you start with a leaky vesicle, that’s hard to test for.
Nonetheless, we spent a happy hour looking at the GPMVs that she had created from the different cell lines. One of the interesting things for me was not only to see the GPMVs themselves, but also to see the very different appearances of the different cell lines under the microscope.
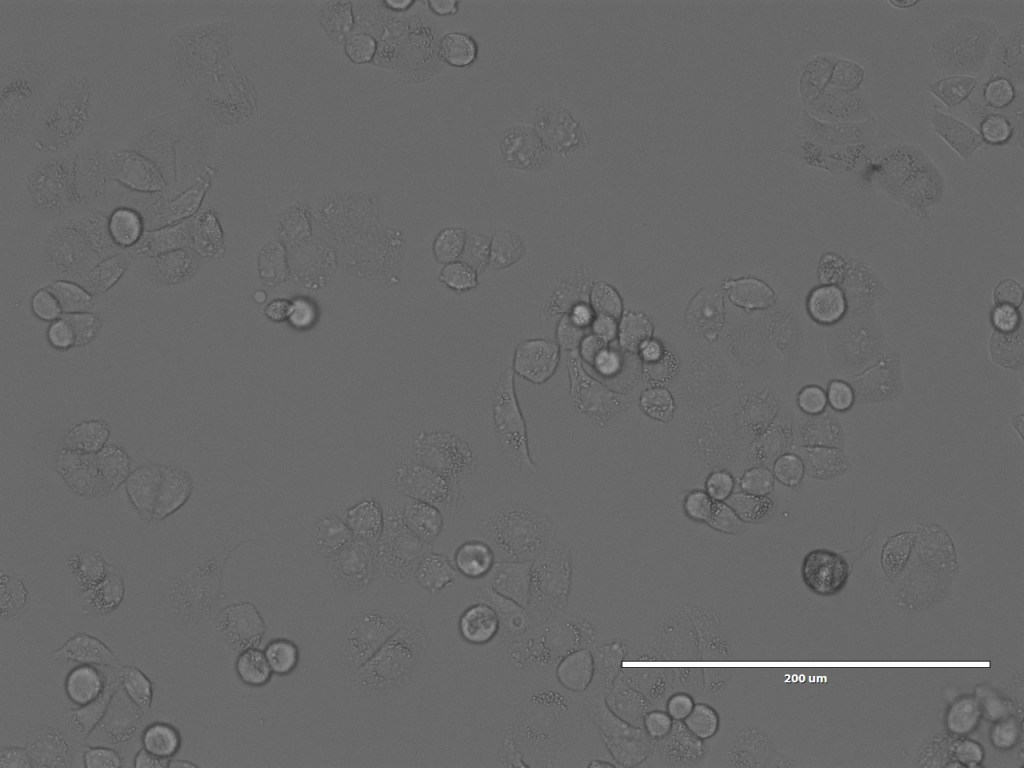
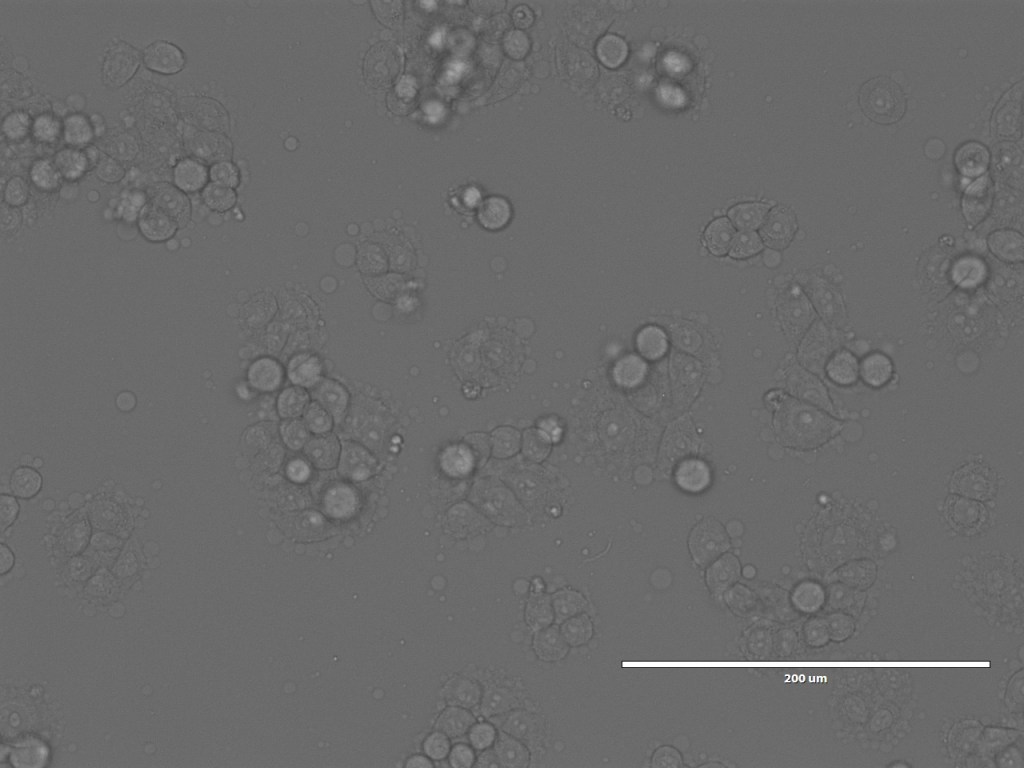
So that brings us more or less to the end of the trip. I did also spend a bit of time with Chris and Debbie in the Bexley Wing and Clinical Research Facility looking at the potential spaces for the piece, but will post separately about that.
Overall it was a wonderful, stimulating visit. I am still bursting with ideas for tests and approaches to making the artwork, even though everything has been disrupted by the Coronavirus spread and the lockdown, which, frankly, has made it much harder to focus on this, or indeed anything.
Stunning images. Why on earth didn’t you become a research scientist? I think you have missed your calling.
LikeLike